What is CPNP?
What is CPNP?
1. What is CPNP? And what is its function?
The Regulation EC 1223/2009 demands pre-market notification. The Notification can only be completed by the designated Responsible Person and only after the Product Information File encloses all evidence of conformity. And the cosmetic products notification portal (CPNP) is a free of charge online notification system created for the implementation of Regulation (EC) No 1223/2009 on cosmetic products.
When a product has been notified in the CPNP, there is no need for any further notification at national level within the EU.
And the CPNP is accessible to competent authorities, European poison centres, cosmetic products responsible persons and distributors of cosmetic products.
The CPNP is making this information available electronically to
competent authorities (for the purposes of market surveillance, market analysis, evaluation and consumer information)
poison centres or similar bodies established by EU countries (for the purposes of medical treatment) CPNP
2. What kind of information do we need to be submitted to CPNP?
The information to be submitted to the CPNP includes:
The category of cosmetic product, its physical form, packaging type and its name or names, enabling its specific identification
The name and address of the Responsible person where the Product Information File is made readily accessible
The country of origin in case of import
The member state in which the cosmetic product is to be placed on the market
The contact details of a physical person to contact in case of necessity
Product formula (frame formulation may be used as well)
The name and the Chemical Abstracts Service (CAS) or EC number of substances classified as carcinogenic, mutagenic or toxic for reproduction (CMR),
of category 1A or 1B, under part 3 of Annex VI to Regulation (EC) No 1272/2008
The product formula or the frame formulation allowing for prompt and appropriate medical treatment in the event of difficulties
The original labeling, and, where reasonably legible, a photograph of the corresponding packaging
3. How to request access on CPNP
3.1 Have a valid EU LOGIN account
The process occurs in 3 steps:
Open the EU Login - Sign in page:
The web link (url) to EU Login is: EU Login
CPNP does not support social networks (e.g. Facebook, Twitter, etc.). Signing it does not require creating an EU Login account.
Enter your email address and click 'Next':
Please note that logging in with your EU Login username is no longer possible. Only your email address can be used.
Enter your EU Login password and click 'Sign in':

CPNP does not support authentication methods other than 'Password' (e.g. mobile phone, token, etc.).
3.2 Be defined as an organization in SAAS
Go to SAAS and click Request Access:
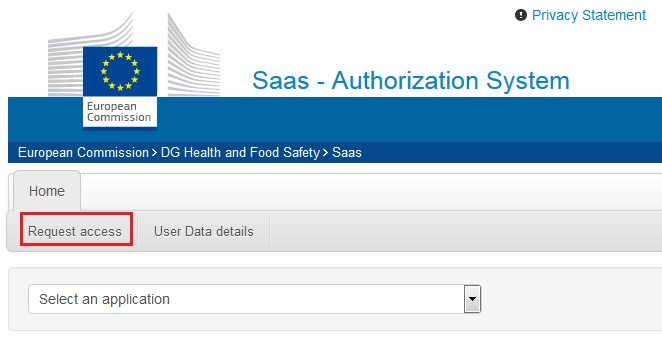
You need a valid EU Login account to be able to request access to CPNP
EU Login formerly known as 'ECAS') is a tool used for authentication to applications and services offered by the European Commission; SAAS is a tool to give authorization on DG GROW's application.
The web link (url) of SAAS is: SAAS - Request access
Select Cosmetic Products Notification Portal in the application list:
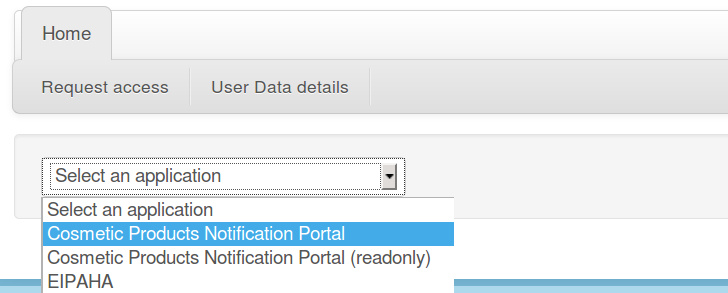
Please notice that SAAS also manages authorization requests for other applications. If you choose another application than CPNP, your request will be ignored or rejected by SAAS.
Click Request access:
Click Next step, step 2: select an existing organisation OR Next step, step 2: create organisation:
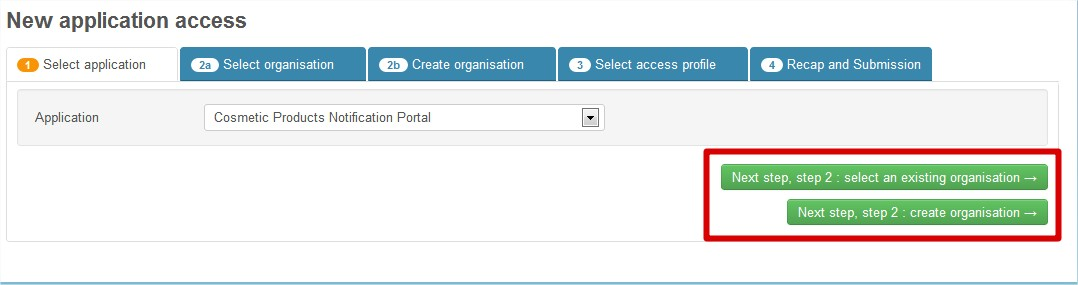
If you are not sure whether or not your organisation exists, choose Next step, step 2: select an existing organisation: you will be able to search for your organisation and create a new one if necessary.
Look for your organisation by typing its name in the Search box:
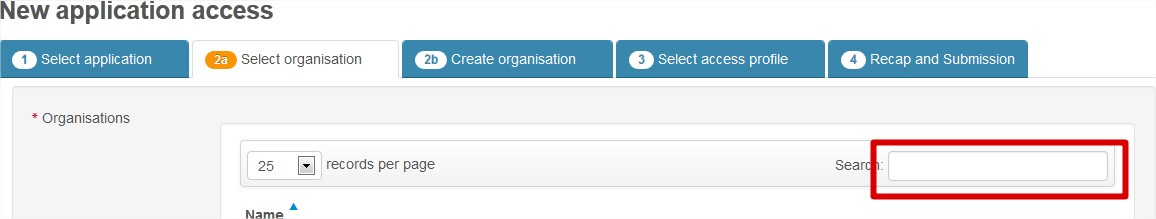
Your organisation is not found and you need to create it.
Select your organisation
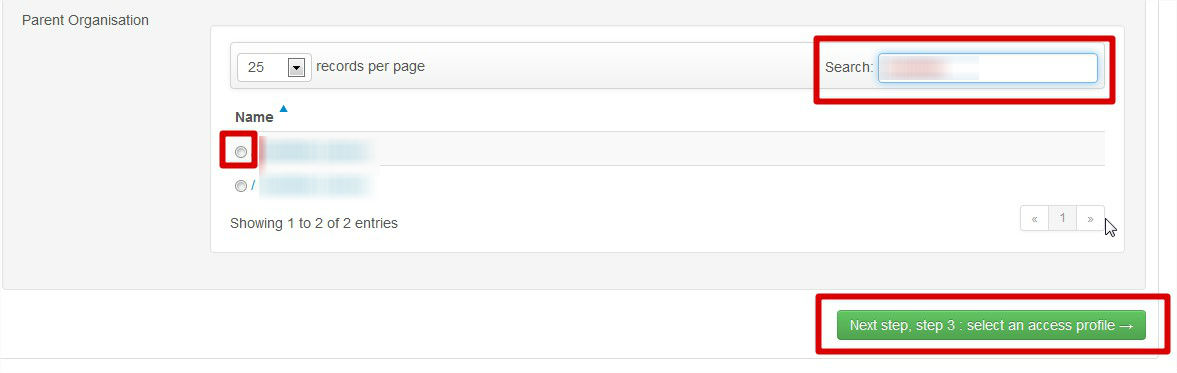
You can choose only one organisation. If you want request access to more than one, you must make a new request per organisation.
Choose one Access Profile by clicking on it and click on the button for the next step
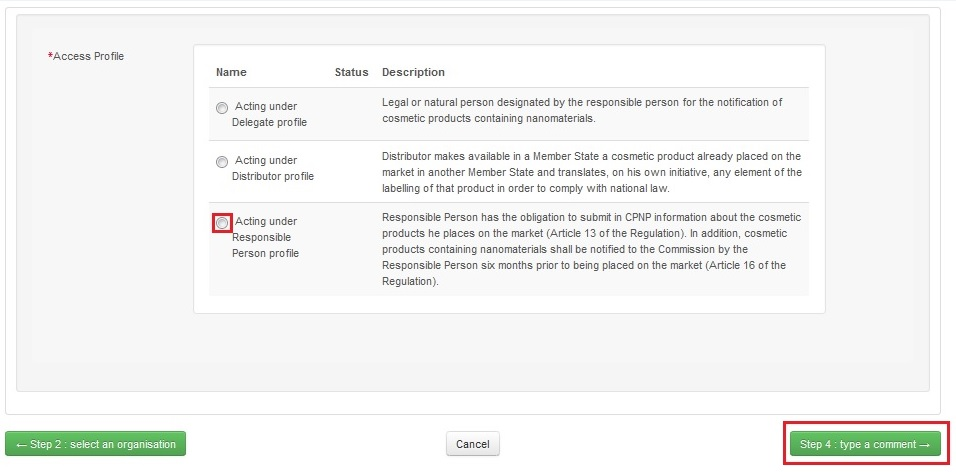
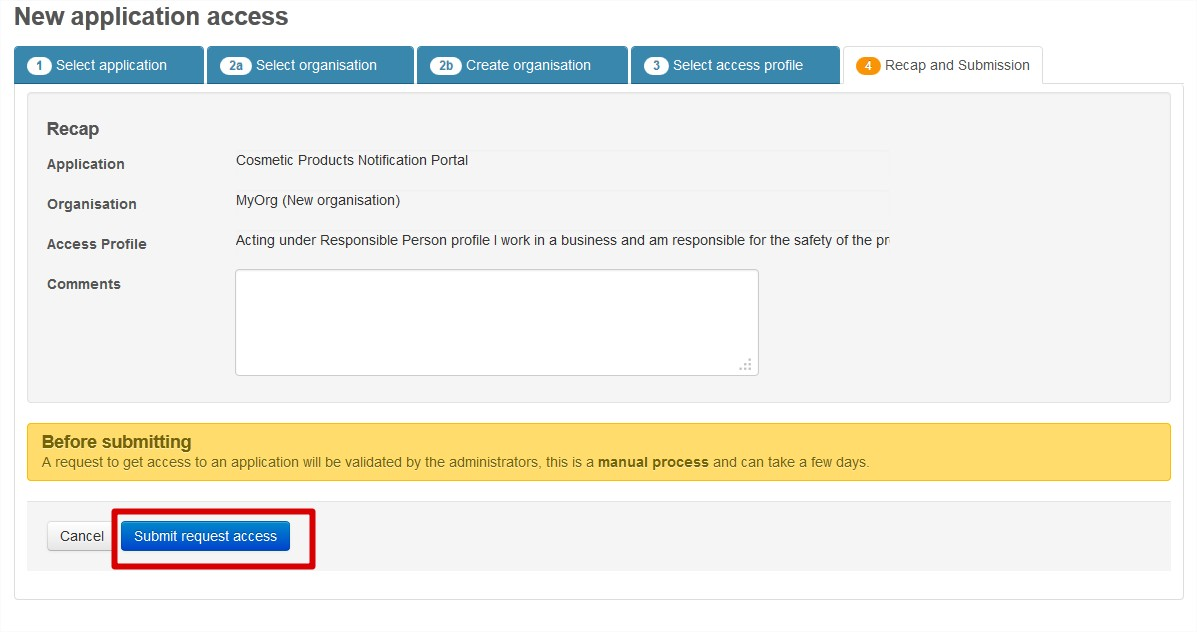
Submit your request
3.3 Enter CPNP
At the completion of the Notification procedure, each product is assigned a CPNP reference code.
4. FAQ
1. What impact did Brexit have on my application? Do I need to prepare other documents?
You are invited to carefully consult this notice to stakeholders on cosmetic products about Brexit and its effects.
2. Notification When Changing EU Responsible Person
Each product Notification (and corresponding CPNP #) is tied to the EU RP which completed the Notification on behalf of the manufacturer. If, at a later date, a brand owner elects to change EU Responsible Persons, the products must be DE-Notified by the previous EU Responsible Person and re-Notified upon the due diligence activities of the newly appointed EU Responsible Person. The previous EU Responsible Person information must also be removed from the packaging. Any future inquiries related to the product compliance will be forwarded to the newly appointed EU RP.
3. Do I need to register my products if I want to sell them in the European Union?
Yes. Regulation (EC) N° 1223/2009 (Article 13) states that all cosmetic products that will be placed on the market in the European Union need to be registered into CPNP prior to being placed on this market.
4. Notification of a new product: when do I have to notify it?
You have an obligation to make this new product notification before placing it on the market. Regulation (EC) N° 1223/2009 does not specify a particular moment, just before putting it on the market.
However, for the notification of products containing nano-materials (Article 16), you must do it 6 months prior to placing them on the EU market.
5. Will I receive a confirmation when I notify a product?
No, no confirmation is sent.
Please note that the fact that a product has been successfully notified through the CPNP does not necessarily mean that the product in question fulfills all the requirements of the Regulation (EC) N° 1223/2009.
6. How can I check if a company has notified its products in the CPNP?
The CPNP is not open to the public. Its access is restricted to the Competent Authorities (for the purposes of market surveillance, market analysis, evaluation and consumer information) and to the Poison Centres or similar bodies established by Member States (for the purposes of medical treatment) and is not available for consultation by the public.
7. We manufacture cosmetic products for different customers. How do we notify their products?
The obligation to register a cosmetic product in the CPNP falls on the Responsible Person that puts it in the market.
Each one of your customers selling cosmetic products under their own name or brand has the obligation to notify the products under its company name.
8. For more detailed Q&A, please visit CPNP FAQ
